---
AWKN-001
About AWKN-001
AWKN-001
AWKN-001 is a first-of-its-kind combination therapy combining IV ketamine (an NMDA receptor antagonist) with copyrighted manualized relapse prevention cognitive behavioral therapy (CBT) targeting both the biological and psychosocial aspects of AUD.
Clinical evidence:
Phase 2 results demonstrated a 50% reduction in Heavy Drinking Days (HDD) vs to placebo
Long-Term Impact: 86% abstinence on average sustained for 6 months post-treatment, compared to just 2% pre-trial
Clinical Development & Regulatory Pathway:
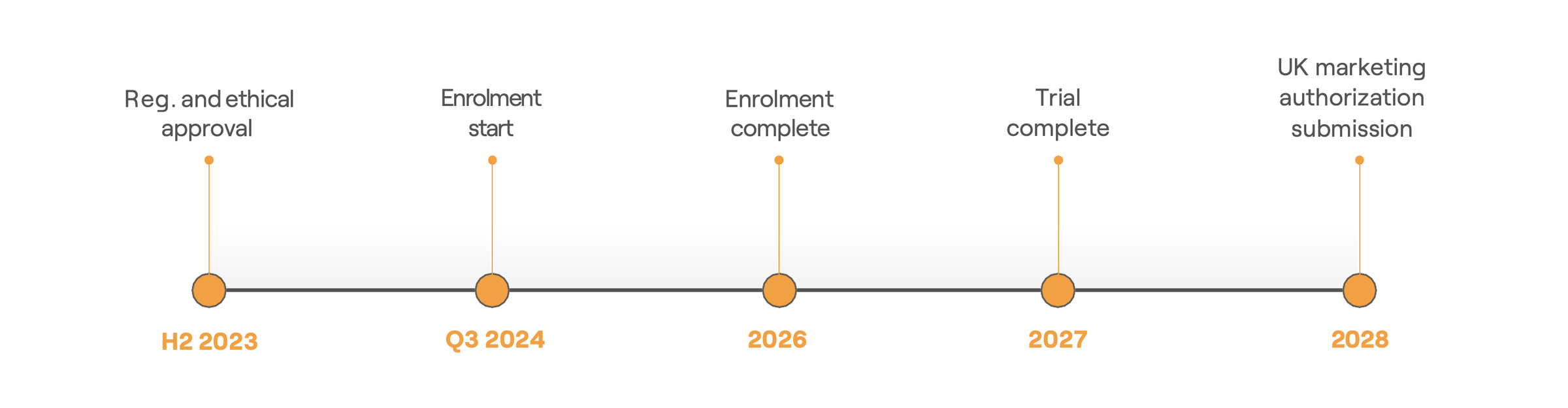
AWKN-001 is in phase 3. The phase 3 trial (“More Kare”) is an n=280 two armed active placebo controlled trial. It is co-funded by the UK National Institute for Health Research (NIHR) Efficacy and Mechanism Evaluation Programme (NIHR150193) and Awakn. It is being run by the University of Exeter Clinical Trials Unit.
Targeting a Regulation 52b hybrid application in the UK for approval as a new indication – provide 8 years data exclusivity and 2 additional years market protection
AWKN-001
AWKN-001 TIMELINE
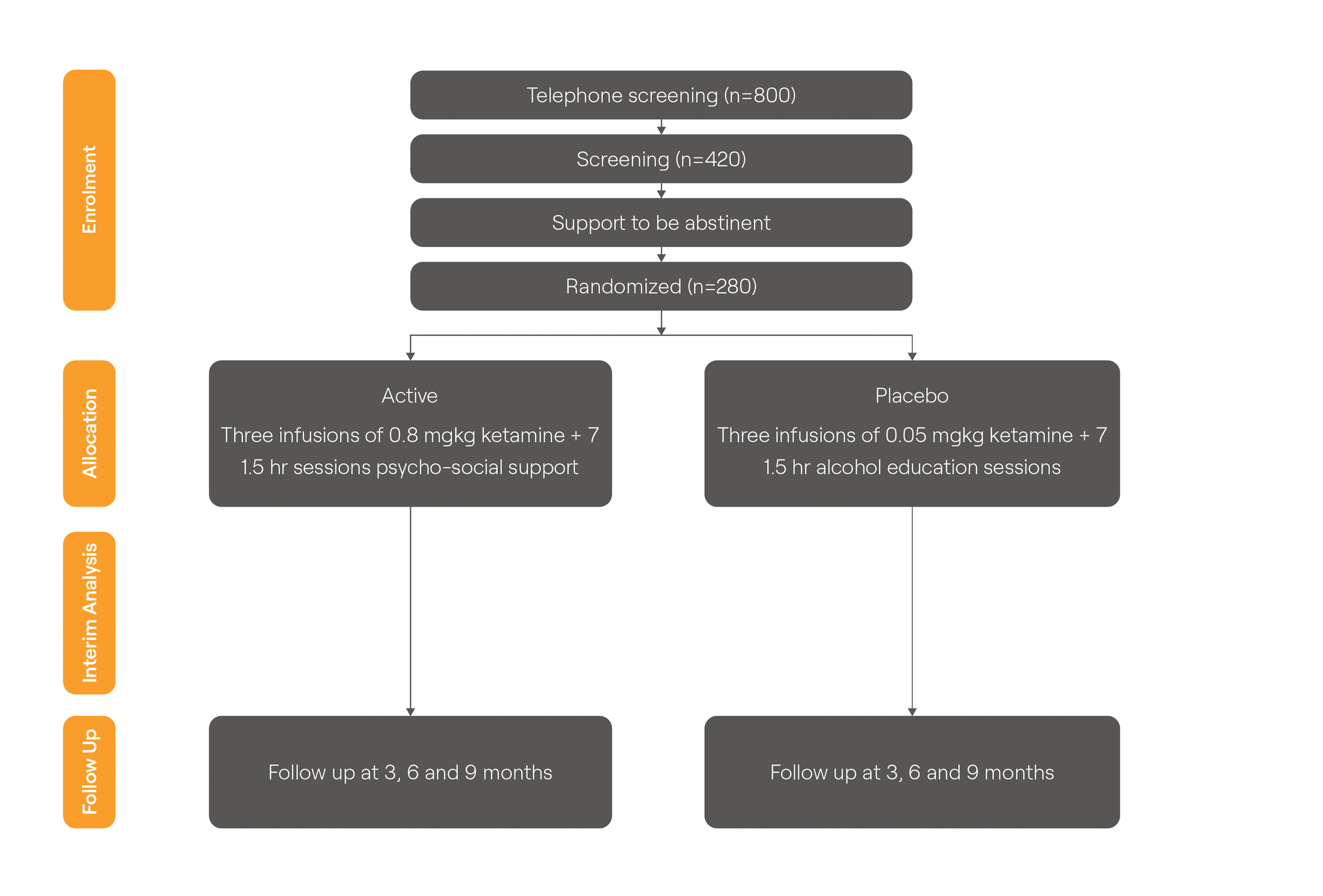
AWKN-001 Ph3 Trial Design
- • n=280 two-armed placebo-controlled trial
- • Trial co-funded by UK Dept of Health and Social Care
- • Cost to Awakn of Phase 3 capped at only £800,000
- • Nine NHS Trust Sites
- • Trial started Q3 2024
• MHRA innovative passport (ILAP) awarded
AWKN-001 Phase III Trial Design
Sign Up for Newsletter
MAIN OFFICE
Awakn Life Sciences Corp
301-217 Queen St. W
Toronto, Ontario M5V 0R2
Canada
© 2023 Awakn Life Sciences Corp | Privacy Policy | Disclaimer