---
Overview
Therapeutic Development Pipeline
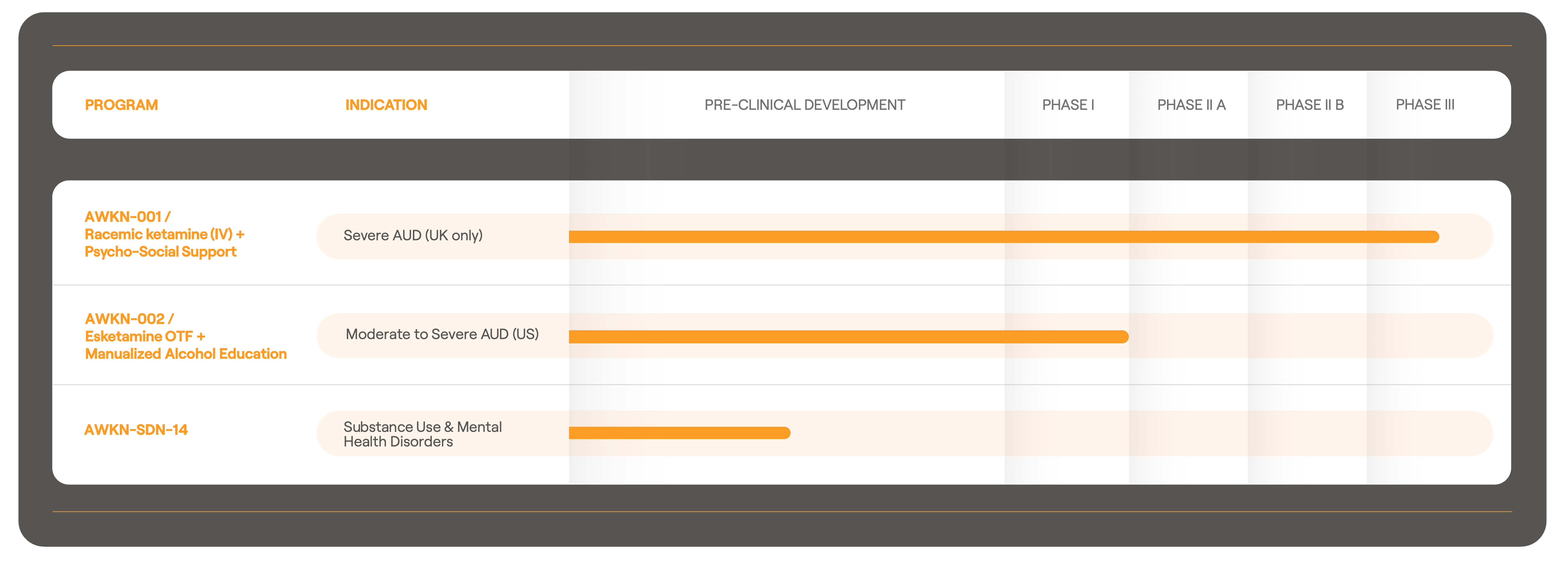
AWKN-001
AWKN-001 is a first-of-its-kind combination therapy combining IV ketamine (an NMDA receptor antagonist) with copyrighted manualized relapse prevention cognitive behavioral therapy (CBT) targeting both the biological and psychosocial aspects of AUD.
Clinical Evidence:
Phase 2 results demonstrated a 50% reduction in Heavy Drinking Days (HDD) vs to placebo.
Long-Term Impact: 86% abstinence on average sustained for 6 months post-treatment, compared to just 2% pre-trial
Clinical Development & Regulatory Pathway:
AWKN-001 is in phase 3. The phase 3 trial (“More Kare”) is an n=280 two armed active placebo controlled trial. It is co-funded by the UK National Institute for Health Research (NIHR) Efficacy and Mechanism Evaluation Programme (NIHR150193) and Awakn. It is being run by the University of Exeter Clinical Trials Unit.
Targeting a Regulation 52b hybrid application in the UK for approval as a new indication – provide 8 years data exclusivity and 2 additional years market protection.
AWKN-002
AWKN-002 is a novel combination therapy consisting of a patent pending proprietary esketamine oral thin film (OTF) with manualised psycho-social support to treat moderate to severe AUD in the US.
Clinical Evidence:
Ph1 was successful completed by LTS Lohmann and in-licensed by Awakn.
Clinical Development & Regulatory Pathway:
In Phase 2b planning.
Positive Pre-Investigational New Drug Application (IND) meeting with FDA in December 2024, FDA supportive of 505(b)(2) pathway.
AWKN-SDN-14
Overview:
AWKN-SDN-14 are a series of serotonin, dopamine, and noradrenaline modulators under pre-clinical investigation by Awakn. Being assessed for potential to promote pro-social behavior and offer an improved safety profile compared to existing treatments being developed for PTSD.
Pro-social behavior is increasingly recognised as a key factor in addressing PTSD. Enhancing these behaviors may help individuals with PTSD overcome feelings of isolation, rebuild interpersonal relationships, and engage more effectively in therapeutic interventions.
Patents
Sign Up for Newsletter
MAIN OFFICE
Awakn Life Sciences Corp
301-217 Queen St. W
Toronto, Ontario M5V 0R2
Canada
© 2023 Awakn Life Sciences Corp | Privacy Policy | Disclaimer